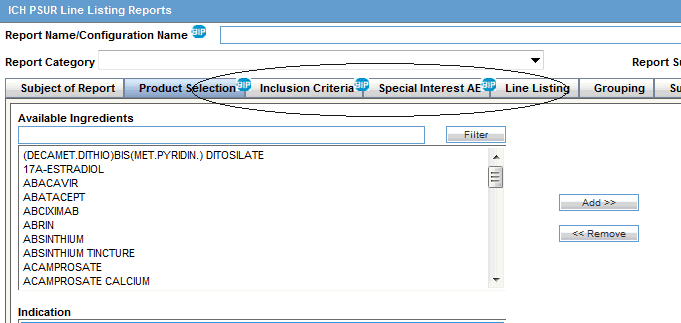
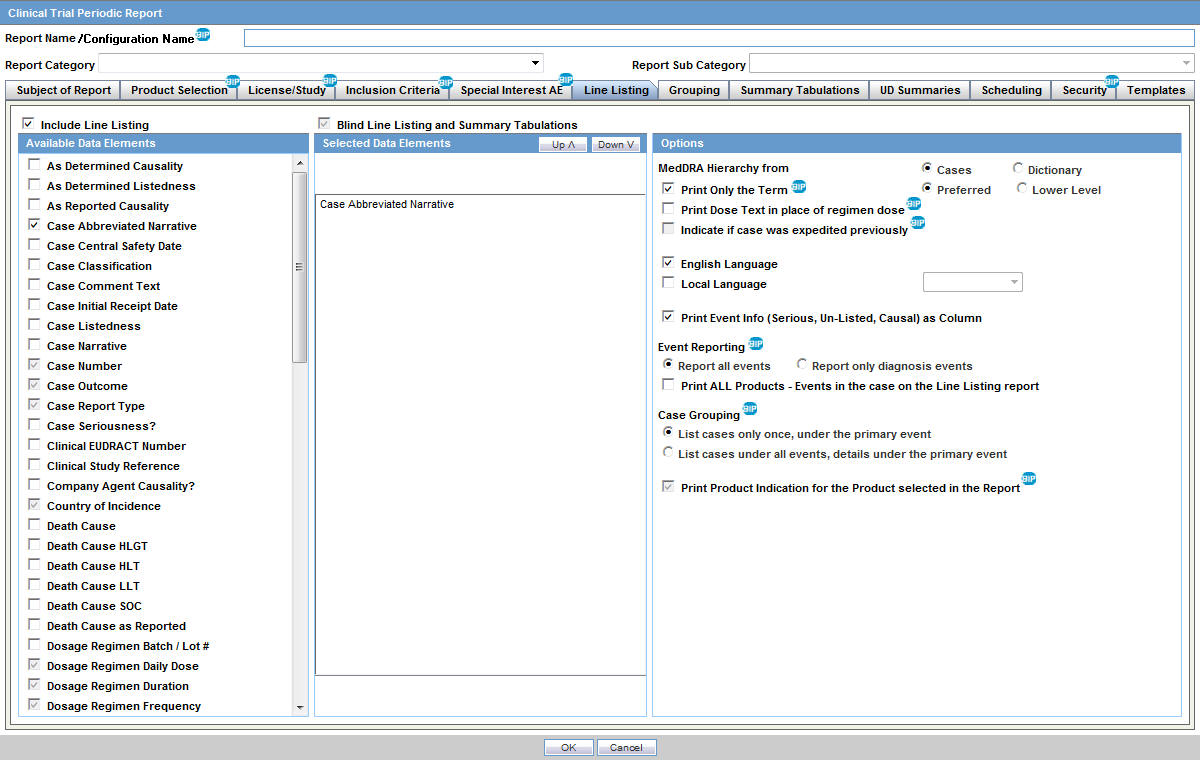
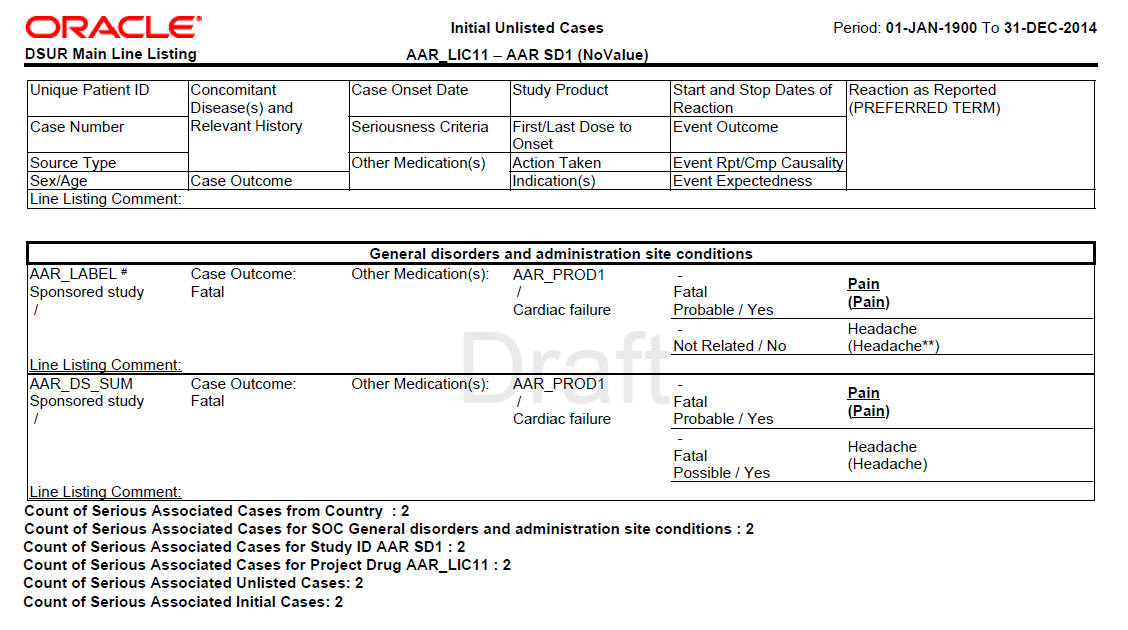
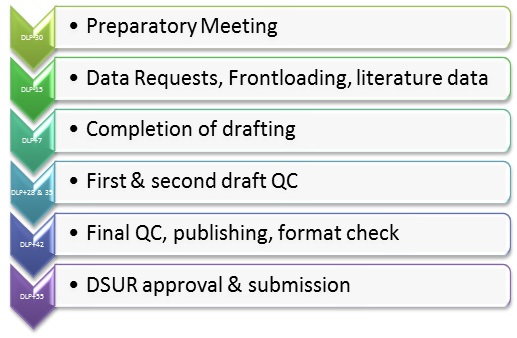
Amazon.co.jp - Development Safety Update Report Dsur, Harmonizing the Format and Content for Periodic Safety During Clinical Trials (Report of Cioms Working Group) | Council for International Organizations of Medical Sciences |本

9789290360803: Development Safety Update Report (DSUR): Harmonizing the Format and Content for Periodic Safety Reporting During Clinical Trials: Report of CIOMS Working Group VII - AbeBooks - WHO: 9290360801

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

TRI Tribune Fall 2012 - The Next Regulatory Challenge: Switching from the IND Annual Report to the DSUR